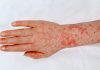
The technique scientists use to separate mixtures is called chromatography. Included in the term chromatography is a range of differing techniques including gas chromatography (GC), thin layer chromatography (TLC) and high performance liquid chromatography (HPLC).
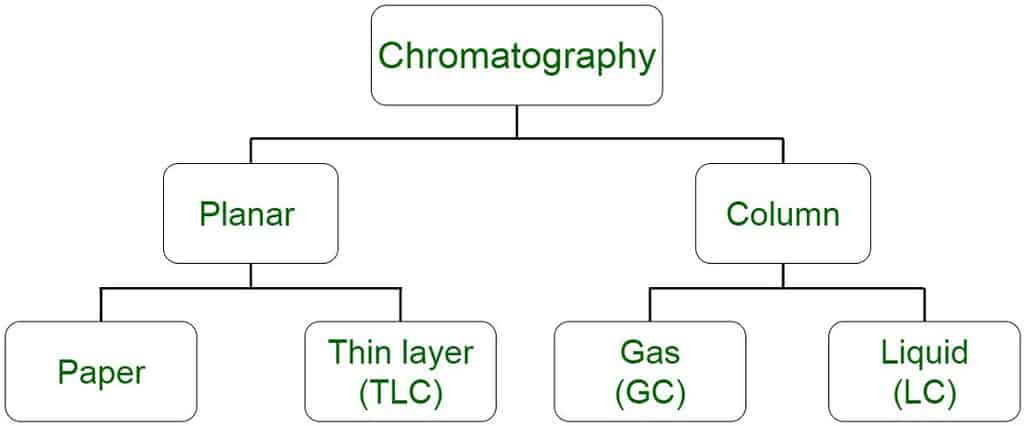
Stationary and mobile phase
All forms of chromatography have a stationary phase, through which a mixture dissolved in a mobile phase is passed. The mobile phase is usually a liquid or a gas which transports the mixture to be separated through a column or flat sheet which has a solid stationary phase.
The mixture is separated because the different constituents are slowed down by the stationary phase by different amounts, a phenomenon known as the partition coefficient.
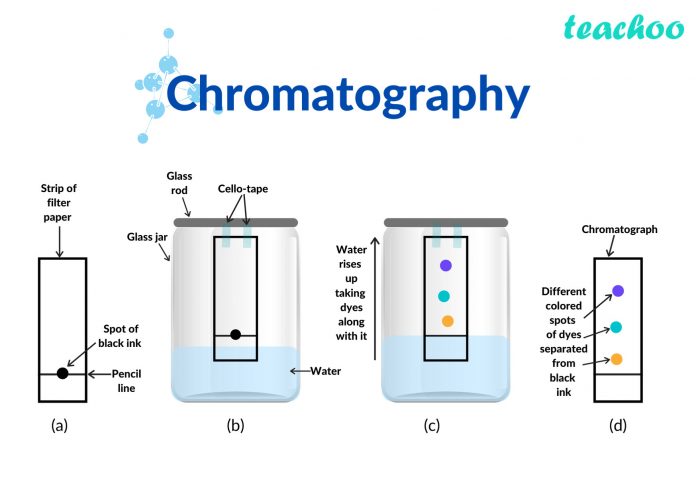
Separation Ink using Paper Chromatography
The simplest type of chromatography can be done using a piece of absorbent paper on which a small dot of the sample mixture is placed. The bottom edge of the paper is then placed in a solvent and, by capillary action, the solvent rises up across the paper, taking the sample mixture with it and separating it.
For example, if a spot of washable black ink is used, water will separate it into a range of different colours, demonstrating that black ink is made of a mixture.
Chromatography Columns
More sophisticated chromatography techniques involve the use of columns, which are long tubes, packed with a solid stationary phase, or capillary tubes with the stationary phase adhered to its wall.
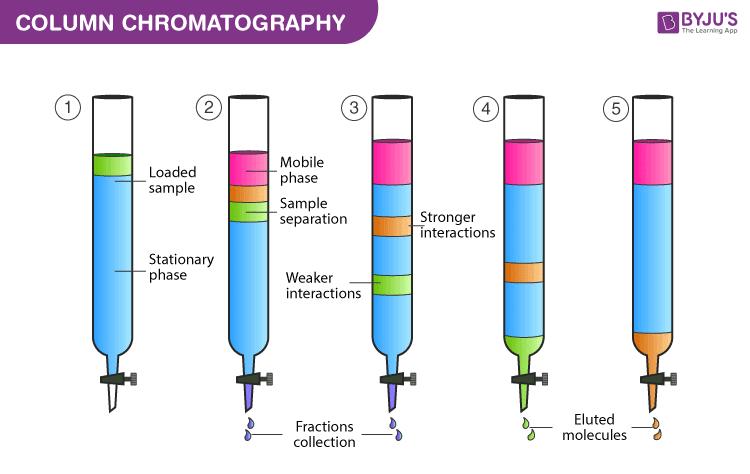
The column can then be placed in an oven to maintain a constant temperature, or, alternatively, to provide a temperature gradient to improve the separation of different compounds.
- What Is Aromatherapy Vs. What Are Essential Oils?
- What is La Tomatina in Bunol, Spain Like? What to Expect at the Famous Tomato Throwing Festival
Gas Liquid Chromatography
Gas chromatography and gas-liquid chromatography use columns packed with an inert solid material coated with the liquid stationary phase. The sample mixture is introduced into the column in a stream of inert gas, which could be helium, nitrogen, argon or carbon dioxide.
At the other end of the column there is a detector connected to a readout, which is chosen depending on the makeup of the sample.
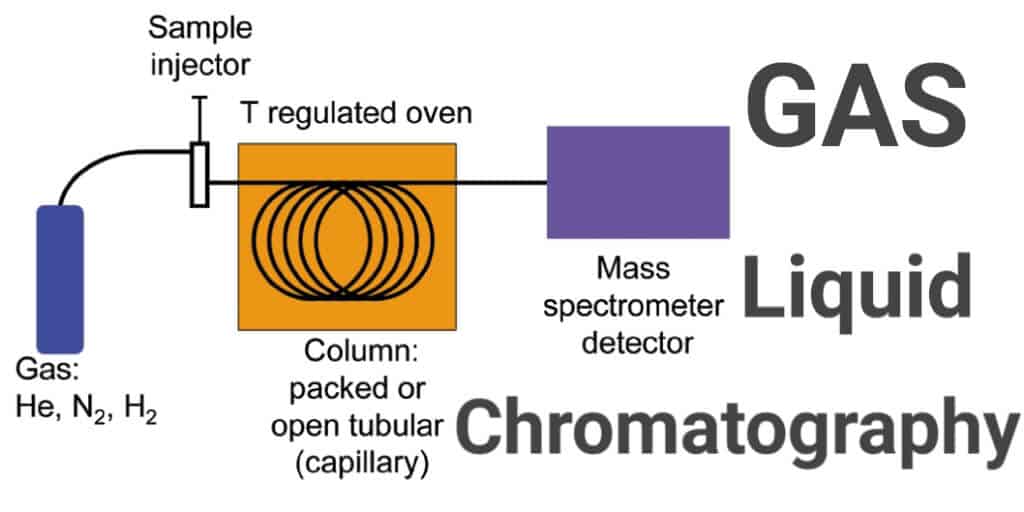
Examples of detectors include the Flame Ionisation Detector (FID) which detects most organic compounds, and a Thermal Conductivity Detector (TCD) which detects the presence of almost any chemical. Gas chromatography is often combined with mass spectrometry, which is able to determine the exact identity of the constituents of a sample mixture.
High Performance Liquid Chromatography
Liquid chromatography uses a liquid mobile phase to transport the sample mixture.

High Performance Liquid Chromatography (HPLC) is often used by biochemists to separate amino acids and proteins due to their different behaviour in solvents related to the amount of electronic charge of each one.
Analytical Chemistry, Biochemistry and Forensic Science
Chromatography is an indispensable tool for many branches of science, especially analytical chemistry and biochemistry.

Forensic scientists also make use of the technique to identify the constituents of samples found in a crime scene.